Multiple Sclerosis
Author: Jany Denisse Rodriguez
An Introduction to the Immune Cells Involved in the Progression of MS
Dendritic cells are antigen presenting cells that play a major role in the initiation of the adaptive immune response
-
It is thought that dendritic cells may collect the antigen from the CNS and migrate to the nearest lymph node where they will present to naïve CD4+ and CD8+ T cells via MHC molecules. After this initial activation, T cells migrate to the CNS where they will differentiate into IFN-γ producing Th1 cells and/or IL-17 producing Th17 cells. These cells will then secrete pro-inflammatory cytokines, initiating the inflammatory reaction which may consequently result in demyelination. The initial demyelination or oligodendrocyte damage will activate microglial cells. Activated microglial cells are capable of cross-presenting antigens via MHC molecules and activating T cells within the CNS. In addition, they release pro-inflammatory mediators that alert CNS resident cells of the tissue damage that is occurring. This further adds to the inflammation within the CNS.
Macrophages are phagocytic cells capable of killing infected cells and tissues. They can also act as antigen presenting cells within the central nervous system in MS.
Helper T lymphocytes (Th cells): activated Th cells enter the CNS and are responsible for producing immune system mediators (cytokines) in response to antigens displayed on antigen presenting cells
-
Th1 cells and Th17 cells have been found in MS lesions
Cytotoxic T lymphocytes (T cells) enter the CNS and cause tissue destruction
B lymphocytes (B cells) are antibody producing cells. In response to antigens, they mature into plasma cells that secrete antigen-specific antibodies
Neutrophils are phagocytic cells capable of destroying infectious cells and tissues. Unlike other immune cells, neutrophils are only present in the CNS when infection or tissue injury is present; they are absent in healthy CNS
Natural killer cells (NK cells) are a type of white blood cell known best for their ability to kill virally infected cells and detecting cancer cells. They are called “natural” killer cells because they don’t need to be activated by antigen prior to performing their duties. They simply attach to every cell they encounter, regardless if it will kill it or not, and recognizes certain molecules on the surface of those cells, that will either cause the NK cell to attack it or leave it alone.
Microglial cells are resident macrophages of the CNS. Microglia have regulatory functions within the CNS, constantly scanning and detecting infections or tissue injury. Upon activation by inflammation, microglial cells become phagocytic cells and act by eliminating the infected cells and their debris.
Astrocytes are the most abundant cells in the brain that provide an optimal environment within the central nervous system. Astrocytes are like the “mother” of the central nervous system, providing homeostatic functions such as potassium balance, blood brain barrier regulation, myelination, neurotransmitter uptake and duration of action, and synapse formation and function.
Endothelial cells and pericytes line the blood vessels of the brain and spinal cord and are the main cells involved in the formation and maintenance of the blood brain barrier.
Neurodegeneration in MS
Neurodegeneration of demyelinated axons is a major cause of the irreversible neurological disability in MS. Healthy neurons are very efficient at conducting nerve impulses. Because of their intact myelin sheath, nerve impulses are conducted in a fast series of action potentials from one Node of Ranvier to another by saltatory conduction. With demyelination present, action potentials are conducted in a wave-like fashion which requires increased expression of Na+ channels along the axon. To restore conduction, the neuron increases its expression of Na+ channels which causes a greater influx of Na+ and increased activity of the Na+/K+ ATPase pump which is used to pump out excess Na+. The ATPase is the largest consumer of ATP within the CNS. To meet the increased amounts of Na+ needed to be pumped out by ATPase, the axons compensate by increasing their numbers of mitochondria.
Since there is a constant outflow of H2O2 from the mitochondria into the cytosol, more mitochondria mean more H2O2. As a result, mitochondria in demyelinated axons suffer from oxidative stress as they try to compensate for the increased need for O2 and ATP. It has been noted that demyelinated neurons turn to glycolysis to meet the increased needs for ATP. Increased levels of lactate, a metabolite of glycolysis, has been discovered in active MS lesions.
In the long run, the increased numbers of mitochondria and the use of glycolysis is not enough to meet the energy demands of demyelinated axons. Without enough ATP to power the ATPase Na+/ K+ pump, neurons suffer from Na+ accumulation, and at high enough concentrations reverse action of the Na+/Ca2+ exchanger occurs, leading to excess Ca2+ influx and Ca2+ induced neuronal cell death. An MRI study documented that Na+ levels were increased twice as much in MS lesions compared to healthy tissue.
Pathway Involved in the Pathogensis of MS
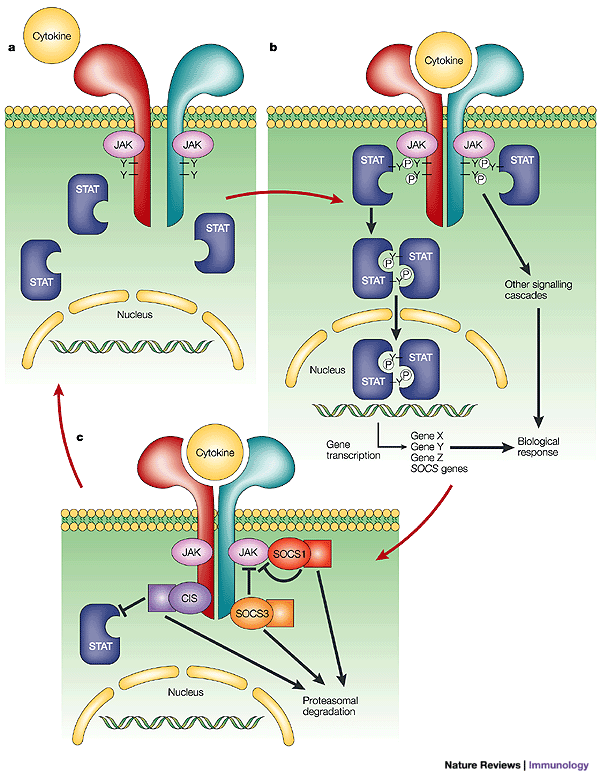
The Janus Kinase/Signal Transducer & Activator of Transcription (JAK/STAT) signaling pathway is the primary signal transduction cascade used by cytokines and is responsible for initiating innate and adaptive immunity and ultimately terminating the inflammatory and immune responses when no longer needed. Dysregulation of this pathway results in autoimmune diseases, including MS. The JAK/STAT pathway is persistently activated in MS because of the excessive cytokine production associated with the disease and the lack of suppressors available to turn off their activity.
Cytokines bind to their receptor and activate receptor associated JAKs (ligand binding brings two JAKs together allowing for transphosphorylation and activation); activation of JAK triggers the recruitment of STATs. When at proximity to each other, JAKs phosphorylate a tyrosine residue on the C-terminus of STATs enabling their activation. Activated STATs dimerize, translocate to the nucleus and induce transcription of target genes. “There are 4 JAKs (JAK1, JAK2, JAK3, and TYK2) and a total of 7 STATs (STAT 1, 2, 3, 4, 5a, 5b, and 6).” [4] The combination of genes expressed depends on which JAK and STAT is activated. Th1 cell differentiation occurs from signaling through JAK2 and TYK2 which subsequently activates STAT1 and STAT4. STAT1 is necessary for IFN-γ signaling and STAT4 is necessary for IL-12 signaling. The combination of STAT1/STAT4 and the cytokines IL-12 and IFN-γ leads to differentiation of naïve T cells into Th1 cells. Th17 cell differentiation requires signaling by IL-6 and IL-23 through JAK1/JAK2 and STAT 3.
Nuclear factor-κB (NF-κB) is a transcription factor that regulates the expression of genes involved in innate and adaptive immunity and inflammatory responses. NF-κB also regulates the activation and differentiation of inflammatory T cells and cytokines. We can see how over-activation of this pathway can also contribute to MS. The constant expression of inflammatory cytokines induced by NF-κB acts as a positive feedback loop for STAT proteins, leading to constant activation of both pathways.
JAKs and STATs are the mediators of almost every signaling event induced by cytokines, and we can see how their persistent activation, as seen in multiple sclerosis, can cause many detrimental effects.
Mutations causing persistent activation of STAT proteins are rare, therefore, their hyperactivity in MS is due to an overabundance of cytokines, dysregulated negative suppressors of JAKs and suppressors of cytokine signaling (SOCS). The SOCS family is composed of 8 members, CIS and SOCS 1-7. SOCS have an SH2 domain and a SOCS box on their C-terminal domain. [4] The SOCS proteins are activated by cytokines and STATs (which stimulate transcription of SOCS genes) resulting in a negative feedback loop that inhibits further cytokine signaling through JAK/STAT. SOCS proteins bind directly to activated JAKs or to the cytokine receptors via their SH2 domains, to inhibit any further JAK kinase activity and signaling. This negative feedback effect controls the duration of cytokine signaling. The SOCS box interacts with components of ubiquitin ligase; ubiquitination targets JAKs for degradation by proteasomes.
Studies have shown that any dysregulation in cytokines, JAKs, STATs, and SOCS proteins can cause excessive activation of STAT3 and STAT4 leading to autoimmune diseases, including multiple sclerosis. T cells and monocytes from MS patients during relapse showed elevated levels of STAT3, compared to patients in remission, and decreased levels of SOCS3. This suggests that increased STAT3 and decreased SOCS3 correlates to MS relapse.
JAK/STAT and SOCS Signaling Pathway
Symptoms Associated with Multiple Sclerosis
-
Nystagmus: involuntary movements of the eyes
-
Diplopia: double vision
-
Blurred vision
-
Visual loss
-
Poor color vision (especially loss of red)
-
Pain upon eye movement (usually on one eye)
-
-
Fatigue, tiredness
-
Involuntary muscle spasms
-
Can affect any limb but most commonly affects the legs
-
Mild stiffness or strong painful spasms
-
Difficulty walking
-
Muscle weakness: could result from under-stimulated muscles or damaged nerves that stimulate normal muscle contraction
-
Balance problems: could feel spinning sensation (vertigo)
-
Numbness or tingling affecting the legs
-
Slurred speech or loss of volume of speech
-
Pruritus: itching that feels like pins and needles
-
Orthostatic hypotension (dizziness upon standing)
-
As the disease progresses, swallowing problems may develop
-
Tremors: upon intention when reaching out to grab something or when moving
-
-
Bladder problems: occurs in at least 80% of MS patients
-
MS lesions block or delay signal transmission in the areas of the CNS that control the bladder and urinary sphincters
-
Could cause a spastic (overactive bladder) or a bladder that does not empty completely and retains some urine
-
Urgency to urinate
-
Prolonged start of urination
-
Nocturia: nighttime urination
-
Incontinence: inability to control bladder
-
Inability to empty bladder completely
-
If left untreated, bladder problems could lead to increased risk of urinary tract infections (UTI) or kidney stones
-
Constipation
-
-
Cognitive problems
-
Difficulty processing information and retaining new information
-
Memory problems
-
Trouble concentrating
-
Planning and prioritizing
-
Emotional instability
-
Anxiety
-
Depression
-
Mood swings
-
Irritability
-
Possible Therapeutic Options
Inhibitors of JAK
Tofacitinib is a selective JAK inhibitor that has been approved for the treatment of rheumatoid arthritis but may be useful in treating multiple sclerosis. It has greatest specificity for JAK3. Since JAK3 acts on cytokines of the γ chain family, Tofacitinib inhibits cytokines IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 cytokines and IFN- γ, but to a lesser extent. What is remarkable about Tofacitinib is that it does not affect any other kinase except JAKs.
Inhibitors of STAT
Although some STAT inhibitors have been introduced, none have been successfully developed because of lack of selectivity and in vivo efficacy. However, it could be achievable by using the following strategies: first by blocking tyrosine phosphorylation at the receptor, next by preventing the SH2 domain on STAT from binding to phosphorylated JAKs, therefore, inhibiting their dimerization, and lastly, interfering with the DNA binding capacity of STATs. By using the current knowledge we have on STAT signaling, there is potential for more research and hopefully future development of successful inhibitors of STATs.
Inhibitors of NF-κB
Mitoxantrone is a cytotoxic molecule that suppresses NF-κB DNA-binding activity and interferes with DNA synthesis and repair. It is administered as a slow intravenous infusion over 5-15 minutes, every three months. “In the recent Phase III trial, both the low (5 mg/m2) and high (12 mg/m2) doses showed efficacy, but the high dose gave the best overall results.” [3]
-
Side effects of mitoxantrone: mitoxantrone holds concerns for cytotoxicity which means the dosage should not exceed 140 mg/m2 and should only be taken for a few years. Other side effects include “nausea, hair thinning, menstrual irregularities, infertility, decreased white blood cells, transient discoloration of urine and sclera.” [3]
Methylprednisolone (a glucocorticoid), used in treating acute relapses of MS, inhibits NF-κB activation in inflamed CNS tissue
Methylthioadenosine prevents the degradation of IκB-α (protein that binds to NF-κB keeping it inactive in the cytoplasm)- preventing the activation of NF-κB
“Epigallocatechin-3-gallate (green tea phenol) exerts protective and regenerative effects in neurons exposed to inflammation, which may be related to its ability to block the activity of 20S/26S proteasome complex, resulting in the accumulation of IκB-α, the suppression of NF-κB activation” [3]
Beta-interferon 1a & 1b
Beta- interferon (IFN-β) is an anti-inflammatory cytokine that would be beneficial for treating MS. IFN-β decreases cell migration into the CNS, inhibits T-cell proliferation, and stimulates the production of the anti-inflammatory cytokine IL-10 and nerve growth factor which may induce remyelination and axon repair. Currently existing are two types of recombinant beta-interferons: beta-interferon 1a (Avonex, Rebif) is a duplicate of human beta-interferon, and beta-interferon 1b (Betaseron) which has 3 molecular differences from human beta-interferon (a likely reason for increased side effects). Beta-intereferon 1a is administered as an intramuscular injection, 30μg one time a week. Beta-intereferon 1b is given subcutaneously, 250μg on alternate days.
Side effects of beta-interferon therapy
-
Interferon- beta 1a: flu-like symptoms, pain at the injection site (intramuscular)
-
Interferon-beta 1b: flu-like symptoms, reactions at the injection site, menstrual irregularities, decreased white blood cell count, elevated liver enzymes
-
Flu-like symptoms can be decreased by initiating therapy at low doses and increasing at a consistent schedule. Concurrently using an anti-inflammatory medication during the first few weeks of therapy may also be helpful.
Two recent studies on the efficacy of interferon beta-1a, CHAMPS and ETOMS, compared the use of therapy with a placebo on patients who recently had their first attack and showed an abnormal brain MRI. These patients were at high risk of MS, but there wasn’t significant data for diagnosis. In both studies, early therapy delayed the onset of a second attack throughout the two-year study period. These patients also showed less disease progression in MRI’s taken throughout the two years of study. After these studies, the National MS Society recommended that therapy be initiated in patients as soon as a diagnosis for relapsing MS is made, because early therapy could substantially lower the rate of disease progression. Subsequent studies showed no significant effect on disease progression in patients with a progressive disease pattern. This indicates the need for beta-interferon therapy during the initial stages when the inflammatory components of MS are in high quantities and can be stopped in their tracks. [5]
Glatiramer acetate
Glatiramer acetate (Copaxone) is composed of amino acid polymers that mimic myelin basic protein, and important factor in CNS myelin. Glatiramer acetate works by activating anti-inflammatory T cells which enter the CNS and inhibit immune responses. It is administered daily as a 20 mg subcutaneous injection.
Side effects of glatiramer acetate: injection site reactions (minor discomfort). "About 10-15% of patients will experience a post-injection reaction: chest tightness, palpitations, flushing, and anxiety a few minutes after injection. This reaction only lasts a few minutes."[5]
The most important thing for patients to know is that therapy for MS is not restorative but it can be preventative of future relapses and additive disability. Additional non-pharmacologic therapies could help enhance the quality of life for a person with MS. Engaging in daily exercises and eating healthy foods packed with good-for-you vitamins and minerals could help improve the symptoms of multiple sclerosis and enhance quality of life.
Mutations causing persistent activation of STAT proteins are rare, therefore, their hyperactivity in MS is due to an overabundance of cytokines, dysregulated negative suppressors of JAKs and suppressors of cytokine signaling (SOCS). The SOCS family is composed of 8 members, CIS and SOCS 1-7. SOCS have an SH2 domain and a SOCS box on their C-terminal domain. The SOCS proteins are activated by cytokines and STATs (which stimulate transcription of SOCS genes) resulting in a negative feedback loop that inhibits further cytokine signaling through JAK/STAT. SOCS proteins bind directly to activated JAKs or to the cytokine receptors via their SH2 domains, to inhibit any further JAK kinase activity and signaling. This negative feedback effect controls the duration of cytokine signaling. The SOCS box interacts with components of ubiquitin ligase; ubiquitination targets JAKs for degradation by proteasomes.
Studies have shown that any dysregulation in cytokines, JAKs, STATs, and SOCS proteins can cause excessive activation of STAT3 and STAT4 leading to autoimmune diseases, including multiple sclerosis. T cells and monocytes from MS patients during relapse showed elevated levels of STAT3, compared to patients in remission, and decreased levels of SOCS3. This suggests that increased STAT3 and decreased SOCS3 correlates to MS relapse.
Impaired SOCS Inhibition: as seen in MS
For our neurons to conduct impulses at the fast pace which is needed, they must be covered in myelin. Oligodendrocytes are cells within the central nervous system that provide support and insulation for axons by forming the myelin sheath. A single oligodendrocyte can myelinate up to 50 axons by extending its many processes. Without the myelin sheath, nerve impulses are slow or impaired. Oligodendrocytes are major targets for immune mediated damage in MS. Therefore, impaired or absent neuronal signaling is hallmark of MS and is associated with the majority of the symptoms experienced.
What is Multiple Sclerosis?
Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS: brain & spinal cord) in which the myelin sheaths covering nerve cells and the oligodendrocytes that myelinate them become major targets for immune-mediated damage. The myelin sheath provides insulation for nerve cells and increases the speed at which action potentials run along the axon. In healthy myelinated axons, the action potential “jumps” from one Node of Ranvier to another, a process known as saltatory conduction (saltatory meaning jumping). Saltatory conduction is a very rapid process and the loss of myelin results in nerve signals being conducted sluggishly or not at all.
Most patients present with a relapsing-remitting pattern of disease: relapses are periodic episodes of new or recurring neurologic symptoms followed by a stable period (remission) of at least 1 month. Relapses produce a continuous progression of disability and over time relapsing-remitting MS will naturally transition to a secondary progressive disease pattern. The secondary progressive pattern of MS is a “chronic and unrelenting progression of fixed disability ,” independent of relapses. It is not possible to predict which form of MS an individual person will have or which symptoms they will experience, but it is possible to determine which cellular processes in their body are being affected. Disruption of the blood-brain barrier that normally protects the brain from unwanted and foreign substances is a cause of active MS lesions. Antigen specific T cells enter the nervous system and upon recognition of their antigen they trigger a cytokine signaling cascade that adds to the damage of the blood-brain barrier. MS lesions result from plaques of demyelination and they come with varying degrees of inflammation depending on the stage of disease. MS plaques can be detected in a contrast enhanced MRI, even in the early “silent” stages when symptoms are not yet evident.
About the Author
This research page on Multiple Sclerosis was authored by Jany Denisse Rodriguez, student at Florida Gulf Coast University in the Bachelor of Science Biology program and member of the National Society of Leadership & Success, FGCU chapter. The semester of Spring 2018 was the toughest but the most rewarding thus far. Starting semester one of the Nursing program and simultaneously taking courses to satisfy my Biology major was a considerable task & a rewarding accomplishment. Since nursing is a research based profession, I was thrilled with the idea of this research project because I knew it would expand my knowledge even further. I consider myself to be a very hard working and ambitious young lady with a drive to succeed that can make even the hardest endeavor attainable. On my free time away from my studies and work, I love to exercise at the gym, or do sprints outside when the weather is favorable. I also enjoy yoga for relaxation.
Without further ado... I truly hope you've enjoyed this web page and find it helpful in your understanding of some of the many mechanisms of multiple sclerosis.
-Jany R